Original URL: https://www.theregister.com/2010/03/15/stanford_scientists_build_safe_lithium_sulphur_battery/
Boffins builds lithium battery that can't explode
Solution: get rid of the metallic lithium
Posted in Science, 15th March 2010 12:09 GMT
Scientists from Stanford University in the US have worked out how to build a lithium-sulphur battery that doesn't require a lithium metal electrode. That, they say, will make it free from the "serious safety issues" that have been holding the technology back.
Lithium-sulphur is seen as a strong contender for the next generation of rechargeable batteries because it can support an energy density far in excess of lithium-ion technology. The upshot: batteries can be made smaller and still support a higher capacity than li-ion equivalents. They're also made from cheaper and less toxic materials.
According to the team - Yuan Yang, Matthew T McDowell, Ariel Jackson, Judy J Cha, Seung Sae Hong and Yi Cu - their lithium metal free battery yields a theoretical specific energy of 1550Wh/kg, four times the 410Wh/kg theoretical specific energy of lithium-ion batteries with lithium-cobalt cathodes.
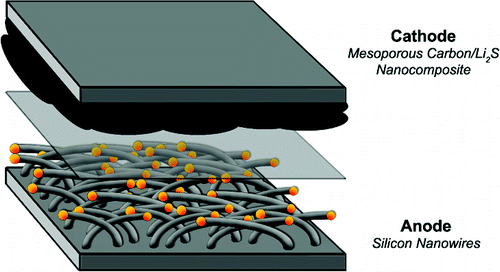
The Stanford battery design
One downside of lithium-sulphur battery design has been the construction of the battery negative electrode. Cathode designs have used lithium metal to help ensure the electrode has sufficient charge capacity. But lithium metal has a tendency to form tree-like crystal structures which can penetrate the the polymer material used to separate the battery's cathode from the positive electrode.
If that happens, the battery short-circuits and that can have literally explosive results.
The Stanford team builds on earlier work in the creation of "mesoporous carbon" electrodes. These are made from tiny, porous carbon rods into which liquid sulphur can be made to flow by capillary action. This key discovery ensures a very good electrical contact between carbon and sulfur, essential to make a workable lithium-sulfur battery design.
However, instead of the troublesome lithium metal, the boffins used lithium sulphide - Li2S - which doesn't promote the formation of battery breaking crystals.
The scientists said they had achieved a discharge specific energy of 630Wh/kg, 80 per cent higher than a typical lithium-ion battery can manage.
But problems remain. The biggest hurdle is the low charge-recharge cycle count: after five discharge and recharge cycles, the Stanford battery's capacity has fallen by two-thirds. After between 40 and 50 cycles, the battery stops holding any charge at all. ®